
SOMMAIRE
LIKE A PANE OF GLASS AROUND THE GLOBE
At its distance from the Sun, Earth should be an ice planet (-18°C). Yet, the average temperature at the globe’s surface is +15°C. Why? Because the atmosphere surrounding us acts like a pane of glass, through which the Sun warms the ground and which prevents this heat from escaping back into space. This is what is known as the “greenhouse effect”.

GASES IN MINUTE, YET ESSENTIAL, QUANTITIES
Water vapor in the air is responsible for two-thirds of the greenhouse effect. However, certain gases, even present in very, very small quantities, also contribute to this effect. These are primarily CO2 (carbon dioxide), methane, nitrous oxide, ozone in the lower atmosphere – a kind of “super oxygen” – and compounds known as CFCs.
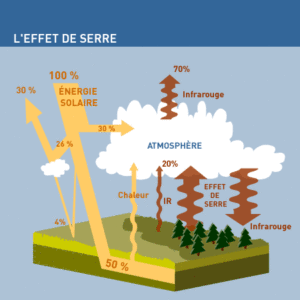
A HEATING SYSTEM AS OLD AS THE WORLD
Since the formation of the solar system 4.6 billion years ago, the greenhouse effect has governed our planet’s climate. However, since its primitive composition, the atmosphere has evolved significantly. In particular, the photosynthesis of the first plants, by gradually lowering the CO2 content, helped maintain a temperature on Earth favorable to life.
The Sun primarily emits electromagnetic waves of short wavelengths (<4µm), including visible light. Part of this energy is re-emitted by the Earth as long-wavelength electromagnetic waves (>4µm): that is, in what is called thermal infrared; because the Earth, like any “hot” body, behaves like a radiator: it radiates into space.
Currently, the solar flux measured at the top of the atmosphere, a few tens of kilometers in altitude, averages 340 watts per m2 (in fact, 1,368 W/m2 on a surface that would be everywhere perpendicular to the light flux: but the Earth is a sphere…). The ground and atmosphere reflect an average of 30% of the solar flux. The rest, approximately 240 watts per m2 absorbed and converted into heat, is used to maintain temperature, set the atmosphere and ocean in motion, evaporate water, etc., thus fueling the planet’s “thermal engine” and regulating climates.
A flux roughly equivalent to the 240 watts per m2 supplied by the Sun is re-emitted into space.
A PHENOMENON AMPLIFIED BY HUMANS
Originally very natural and valuable for Earth’s balance, the greenhouse effect can be amplified if the concentrations of the gases responsible for it increase. And this is precisely what our human activities produce. This increase in the “greenhouse effect” due to our polluting emissions is concerning for the future of our climate.

THE MAIN ACTORS:
> Water vapor (H2O) is produced by the evaporation from plants, soils, watercourses, and oceans. A small quantity is also released by volcanoes (this is how the primitive Earth’s atmosphere was formed).
> Carbon dioxide (CO2), of natural origin, comes from the respiration of living beings (whereas photosynthesis in green plants consumes it), from combustions (fires…) and volcanic eruptions. This presence of CO2 in the air is regulated by the Ocean, which is capable of absorbing large quantities of it.
> Methane (CH4) of natural origin is released by the decomposition of organic matter in oxygen-deprived environments: decay in marshes, mangroves, thawed soils of Arctic regions, or animal digestion. It also escapes during volcanic eruptions.
> Ozone (O3) in the lower atmosphere, of natural origin, presumably descends from the stratosphere via “pockets” pierced by high-altitude jet streams. Ozone is oxygen, but its molecule does not contain 2 atoms, like the dioxygen useful for our respiration (O2), but 3. This ozone in the lower atmosphere (troposphere) is a real poison for plants, skin, and the respiratory system (asthma attacks). At high altitudes, around 20 or 30 km, ozone is, on the contrary, very useful: it protects us from harmful ultraviolet solar rays, dangerous for life; this is the ozone of the seasonal “hole” – a lack of ozone – identified above Antarctica, whose formation worries scientists, as its decrease could have harmful effects on living beings.
> Nitrous oxide (N2O) is one of the products of nitrate transformation by soil bacteria. It then escapes into the atmosphere and reacts to produce nitrogen monoxide (NO).
> As for CFCs, or Chloro-Fluoro-Carbons, these are all compounds emitted by human activities. They are mentioned here for comparison with other greenhouse gases.
THE IMPORTANCE OF GREENHOUSE GASES:
| Gas | Concentration (ppm*) | Greenhouse Effect Efficiency | Contribution to Greenhouse Effect (Watt/m2) |
| H2O (vapor) | 3,000 | 1NBSP. | 100 |
| CO2 | 355 | 1 | 50 |
| CH4 | 1.8 | 21 | 1.8 |
| N2O | 0.3 | 310 | 1.3 |
| O3 | < 0.1 | 2,000 | 1.3 |
| CFC | 0.01 | 10,000 | 0.3 |
* 1 ppm (parts per million) = 1cm3 per m3 of air
PHYSICISTS’ CORNER
In the atmosphere, “greenhouse gases” are present in minute and generally fluctuating concentrations. However, their molecules, formed of 2 or more atoms, vibrate and agitate under the effect of infrared rays reflected by the ground or the ocean, which warms the atmosphere. A colossal “heat trap” that produces a flux of 340 Watts per m2, which is much more than the solar energy directly reaching the ground (158 w/m2)!
When variations of a phenomenon are self-reinforcing, it is called “positive feedback”; when they are self-limiting, it is called “negative feedback.” In the atmosphere, water vapor amplifies variations in the greenhouse effect. Indeed, according to the laws of physics (Clausius-Clapeyron law), the colder the air, the less water it can contain as vapor (formation of fog in cold weather): which reduces the greenhouse effect. Conversely, the warmer it is, the more water the air can contain (hot and humid tropical climates): which strengthens the greenhouse effect.
If, at a given pressure and at -20°C, 1 kg of air can contain 1g of water vapor, at -10°C, it can contain 2g. But at +20°C, this same amount of air can trap 15g of water and 26g at 30°C. Beyond these values, humidity saturation occurs, and droplets or ice crystals form: fog, frost, clouds… or condensation on objects.
JEAN-LOUIS ETIENNE AND ATMOSPHERIC GAS MEASUREMENTS
During the EREBUS mission, Jean-Louis Etienne and his collaborators collected trace atmospheric gases (ozone, methane, etc.) throughout the “descent” from Antarctica towards the southern continent. These measurements were integrated into a study aimed at better understanding the complex chemistry of trace gases in the atmosphere. This photochemistry – so named because it is influenced by the sun – regulates the delicate and still poorly understood balances of our gaseous envelope.


DID YOU KNOW?
The Moon is completely devoid of atmosphere because its mass is too small to retain gases. Therefore, it experiences no greenhouse effect. Venus or Mars, however, which retain a dense atmosphere, experience this phenomenon.
FURTHER READING
BIBLIOGRAPHY
- The Greenhouse Effect (Special Issue, La Recherche No. 243, 1992)
- Educational Dossier: The Arctic and the Boreal Environment (P. Avérous – CNDP, 1995)
- Erebus Expedition (J.-L. Etienne / P. Avérous- Arthaud-1994)
- Educational Dossier “EREBUS”: The Polar Environment 2 (P. Avérous-Autrement dit, CNDP-1994)
- Encyclopædia Universalis
- Earth… Our Planet (P. Avérous-Nathan-1990)
- Atmosphere, Atmosphere (Science & Vie, Special Issue No. 174-1991)
- What Climate for Tomorrow? (S. Huet, Calmann-Levy, 2000)
- The Uncertainty of Climates (R. Kandel, Hachette, 1998)
- Earth’s Climate (R. Sadourny, DOMINOS, Flammarion, 1994)


