Polar Encyclopædia
THE INCREASE IN THE GREENHOUSE EFFECT
THE GREENHOUSE EFFECT – INDISPENSABLE TO LIFE ON EARTH
Like the glass panes of a greenhouse, our planet’s atmosphere reflects back towards the Earth’s surface some of the infrared light (or heat…) that the Earth emits when it is heated by the Sun’s rays. This so-called “greenhouse effect” is a natural phenomenon; without it, our planet would be an icy wasteland at -18°C. Certain gases, even when present in minute traces, as well as dust and clouds can accentuate or diminish this phenomenon
HUMAN ACTIVITY UPSETS THE EQUILIBRIUM
All sorts of human activity – industry, transport, agriculture – modify the composition of the atmosphere, and the gases released can accentuate the greenhouse effect. How does our environment, and particularly the climate, react to this? A number of changes can already be observed, such as a rising sea level and an increase in the average temperature. In an attempt to predict future consequences, increasingly complex climate models are being tested using super-computers.
THE CARBON CYCLE
Each year, the growth and decomposition of plants absorbs and releases in turn about 200 billion metric tons (Gt) of carbon. Human activities release 7 Gt. It has been determined that the Earth’s vegetation and the oceans each absorb about a quarter of this, so the remaining half is apparently accumulating in the atmosphere. And helping to accentuate the greenhouse effect.
WHAT CAN BE DONE ?
The keys to the Earth’s climate are the activity of the Sun and the exchanges between the land, the ocean and the atmosphere. Satellites can be used to take measurements (wind, temperature, cloud cover, vegetation) on a global scale, but numerous different phenomena interact here, and this makes interpretation of these results very delicate. However, there is no doubt that if we reduced the amount of gases being released into the atmosphere it would curb the disruption that has been observed. But action must be taken soon, because it will be years before the results are visible.
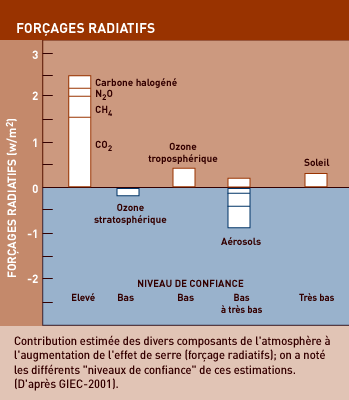
GREENHOUSE GASES (GHG)
The earth’s atmosphere contains a number of gases, present in minute quantities that are usually expressed in parts per million (ppm). These gases are now called greenhouse gases (GHG) because their molecules have the ability to absorb large amounts of the energy that passes through the atmosphere. The main GHG are water vapour, carbon dioxide (CO2), methane (CH4) and nitrogen protoxide.
The greenhouse effect is a natural phenomenon and has existed even since the Earth was formed. But the gases released due to human activities have made it more powerful, either by introducing artificial GHGs that were not previously present or by significantly increasing the concentration of natural GHGs.
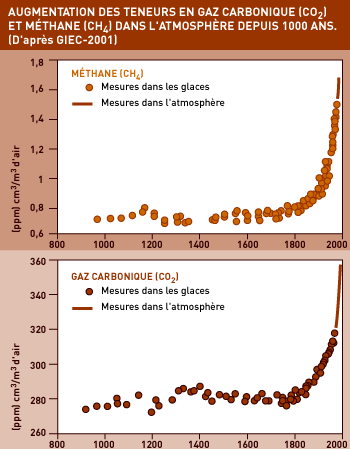
CO2 RELEASED DUE TO HUMAN ACTIVITY
Carbon dioxide is not the only GHG, far from it, but it generates as much greenhouse effect as all other GHGs combined. Before the “industrial revolution”, our atmosphere contained about 280 ppm of CO2. Today, that has risen to 370 ppm, which – if we are to judge by the “climate archives” preserved in the Polar ice caps – is the highest concentration for the last 400,000 years. And climatologists are talking about the possibility of 540 to 970 ppm by the end of the century!
This unprecedented increase is entirely due to gases generated by human activity, and the consequences for the climate are no longer in any doubt.
WHAT ABOUT METHANE ?
Today, methane content in the atmosphere has reached 1.8 ppm (compared with 0.7 ppm in the early Middle Ages), which translates into 5 tons of methane in all. Furthermore, methane content is increasing at a rate of nearly 1% per year – 3 times as fast as CO2. And each molecule of methane has a “warming” power 21 times greater than a molecule of CO2.
Methane is released when organic matter decomposes, and the main sources of gas release are wetlands and rice paddies, but also rubbish dumps and bovines (digestion), etc.
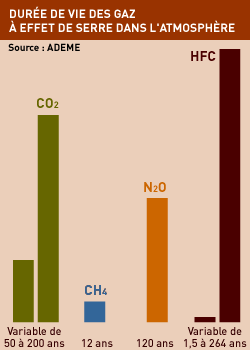
LIFE EXPECTANCY OF THE CULPRIT GASES
The impact of GHGs does not only depend on the amount present in the atmosphere at a given moment but also on how long each gas will remain. The time it takes for half of a given gas to disappear is called its half-life. It takes about 12 years for half of today’s methane to disappear, but the half-life of CO2 is about a century and the half-life of another GHG called CF4 is 50,000 years. Water vapour, on the other hand, disappears after only a few days. This is why even if action were taken immediately to stop GHG emissions, the effects of some existing gases would still be felt for decades to come.
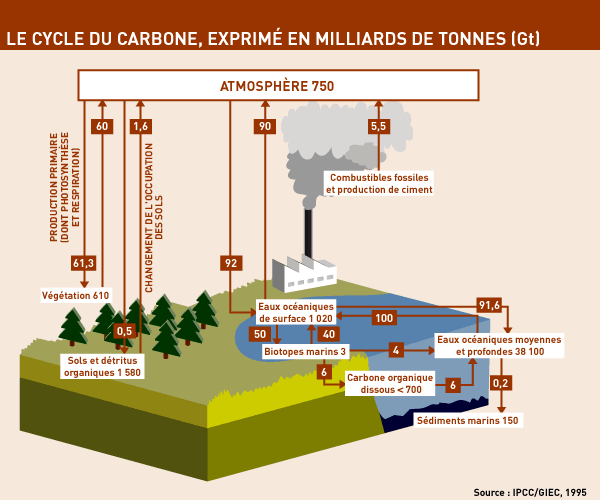
WHAT HAPPENS TO THE CARBON THAT WE GENERATE ?
Scientists estimate that the Earth’s atmosphere contains 750 Gt of carbon, whereas some 39,000 Gt are contained in the oceans (of which 1,000 is in the surface layer) and about 2,200 Gt in the soil and the vegetation. These reservoirs of carbon are immense, and the exchanges between them are correspondingly huge: 60 Gt from vegetation into the atmosphere and vice versa each year (photosynthesis, breathing, etc) and 90 Gt is exchanged between the atmosphere and the ocean.
The gigantic volumes dwarf the amounts generated by human activity: only 5.5 Gt of carbon is released via combustion of fossil fuels (oil, coal, etc.) and 1.6 Gt as a result of agriculture (slash-and-burn operations, etc.).
So what happens to the carbon that is produced as a result of human activities? Can our planet absorb it? Not completely; the Earth seems saturated. Half of the “human” carbon is absorbed by the oceans and terrestrial vegetation (in equal amounts), but the rest – between 3 and 4 Gt – remains in the atmosphere. Nature may be a “carbon sink” but it is not efficient enough to dispose of our excess carbon. And it is this artificial addition to the Earth’s carbon that is accentuating the greenhouse effect and putting the planet’s heating system out of order.
The consequences of this are beginning to be felt (higher temperatures, rising sea level, melting glaciers, etc.) and despite a few remaining uncertainties, experts are almost unanimous in seeing this as a real threat in future.
So how could we dispose of our excess carbon production ?
FORESTS AND CROPS
A first solution seems to be the forests, which act as a “sink” for the carbon in the atmosphere by absorbing the CO2 via photosynthesis. Or at least they do so during periods of growth and extension (biomass increase). But once a forest is mature, fires and rotting vegetation release into the atmosphere some of the carbon stored in plant life. On the other hand, wood used for construction (as long is it doesn’t rot and isn’t burned) does not return the carbon to the atmosphere.
Because trees in their growth phase and the soil absorb part of the carbon in the atmosphere, some people think that we could dispose of the excess carbon by planting new forests. There are two catches here: 1) the carbon stored this way will eventually come back “into circulation” and 2) in order to keep the carbon sink working efficiently the forests will have to be managed (surface area, types of trees, uses of wood, etc.).
As for crops as a solution, admittedly they do absorb a little carbon, but they also indirectly produce methane and nitrogen protoxide, which are both powerful GHGs.
A LONG OCEAN VOYAGE
The oceans contain 60 times more carbon than the atmosphere, in various forms (dissolved in the water, organic matter, minerals, etc.). This immense natural reservoir pumps carbon in two ways:
Firstly, via a purely physico-chemical phenomenon. Seawater can contain dissolved CO2, and the lower the temperature the more CO2 the ocean can hold. This means that the waters in the Arctic are very efficient carbon sinks. Once the carbon is dissolved in the water it can be swept along with the deep ocean currents and may only reappear on the surface 1,000 years later and thousands of kilometres from the region where it was first “captured”. And part of the carbon will remain in the seabed sediment to begin another life cycle.
Another cycle? Because life forms in the ocean pump carbon around as well – via photosynthesis when phytoplankton is in its growth phase, but also via the formation of animal shells and the arrival of organic matter falling from the upper layers of the ocean.
Could we improve the efficiency of this dual oceanic pump? Perhaps, but we must be careful not to play the sorcerer’s apprentice.
WE MUST MAKE AN EFFORT !
Nature does not seem able to absorb the surplus carbon that human activities generate each year, so we must generate less. In order to stay within the limits of what the Earth can recycle (between 3 and 4 Gt), we must reduce our emissions by nearly half. Countries are moving to curb emissions but we are a long way from reducing them by half. Indeed, the Kyoto Summit proposed reducing emissions by only 5%. But as CO2 remains in the atmosphere for nearly two centuries, the consequence of our current emissions are not going to disappear for a very long time…
TO FIND OUT MORE …
BIBLIOGRAPHY
- Changement climatique 2001: impact, adaptation et vulnérabilité – GIEC/IPCC, janvier 2001
- Les dérives du climat : Dossiers & Documents – Le Monde Nov. 2001
- Quel climat pour demain ? S. Huet, Calmann-Lévy, 2000
- Enquête sur le climat passé, enquête sur le climat du futur, Revue du Palais de la Découverte, mars-avril 2001
- L’effet de serre – La Jaune et la Rouge, mai 2000 (Conférences-débats, X-Environnement) Changement climatique : un défi majeur (Ademe, déc. 2000)
Support the project with a donation
The Polar POD expedition is one of the stamp of the pioners, a human adventure coupled with a technological challenge, an oceanographic exploration never before carried out which will mark a milestone in the discovery of the oceans.
Thank you for your support !